Oxidation Reduction Potential (ORP)
What is ORP?
ORP, or “oxidation-reduction potential” (also called “redox potential”), is a measurement of water’s tendency to act as either a reducing agent (electron donor), or oxidizing agent (electron acceptor). A positive ORP indicates the presence of potential oxidizers, while a negative ORP indicates the presence of potential reducers. The measurement is done using an ORP meter, which includes a probe designed to be inserted into the water being tested. The ORP of water produced by ionizers, hydrogen infusion machines (HIM’s), and other hydrogen water technologies (e.g. H2 tablets, sticks, cartridges, etc.), will typically measure some negative value (-100 to -750 mV, depending on pH). Keep in mind that this “measured potential” only indicates the water’s tendency to act as a reducer/oxidizer, and does not guarantee that any particular oxidation/reduction reaction will occur, or indicate the strength or speed of the reaction if it does occur. In order to know whether or not any oxidation or reduction reaction will occur inside the body, the chemistry associated with the reaction in question (kinetics/thermodynamics) must be thoroughly investigated and understood. Where does the negative ORP reading come from and what does it mean?

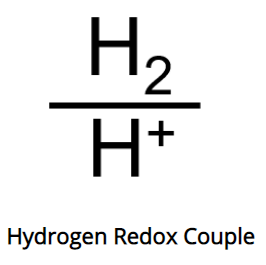
The bottom term, H+, is the ion whose concentration determines how acidic water is, and is the “H” in pH (potential of hydrogen). This is significant, as it tells us that the ORP measurement is influenced by the pH of the water being tested.
(Note: although pH means “potential of hydrogen”, pH represents the concentration of the hydrogen ion, H+, not H2 gas)
Both species of hydrogen contribute to the final ORP reading, producing a small corresponding voltage potential at the electrode (based upon their relative concentrations and # of electrons) contained within the meter’s probe assembly. The meter amplifies and compares the voltage measurement against an internal reference, and displays a digital reading (usually in “millivolts”). However, while the dissolved H2 gas (a reducing agent) is responsible for the negative ORP reading, the magnitude of the reading does not directly correlate with the amount of dissolved H2 gas in the water, and cannot be used as an accurate “measurement” of the dissolved H2 concentration. It is also important to note that, just because water has a negative ORP, this does not necessarily mean the water will have any therapeutic benefit. There are many other compounds (some toxic) which, when dissolved in water, can produce a negative ORP reading. In order to know whether or not there may be a therapeutic benefit, the particular compound producing the negative ORP (redox couple) must be specifically identified. Based on published research, we know that H2 gas, the source of the negative ORP, is a therapeutic agent.
Why do some waters containing dissolved H2 have an alkaline pH, while others are closer to neutral?
Electrolyzers (alkaline ionizers) produce H2 gas at the negative cathode by reducing (adding electrons to) H+ ions to H2 gas using electricity. Because H+ ions are the acid component in water, their consumption during the production of H2 gas elevates the pH of the drinking water into the alkaline range ( above 7pH) as the hydroxide level (OH–) rises. The following equation describes the reduction of H+ ions to H2 gas:
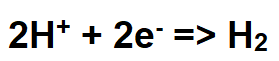
Other types of hydrogen water devices, hydrogen infusion machines (HIM’s), utilize a proton-exchange membrane (PEM) to make H2 water, and do not perform electrolysis directly on the drinking water. Instead, they produce hydrogen gas in a small electrolysis chamber, and then infuse the filtered drinking water with the gas using a special device called a “dissolver”. Therefore, depending on the pH of the hydrogen water being tested, ORP measurements will produce very different (and often confusing) results, making it impossible to use the ORP reading for measuring dissolved H2 levels or for evaluating the therapeutic benefit of the water.
Using the Nernst equation to predict ORP values
In chemistry, the well-known Nernst equation is used to predict reduction potentials (ORP) in a variety of systems. The form of the Nernst equation used for dissolved hydrogen gas is:

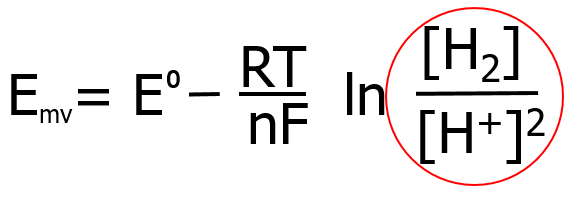
Notice that the H2/H+ redox couple mentioned earlier (circled in red) is contained within the equation (the brackets signify “concentrations”). Therefore, the Nernst equation must include values for the the relative concentrations of each species of hydrogen to predict the ORP. The equation also includes other important chemistry values and variables (water temperature, gas constant, number of electrons transferred, and Faraday’s constant). Using the Nernst equation, along with selected values for pH and H2 levels, we can predict the effect that changes in each will have on the resultant ORP measurement. These predictions can then be used to analyze and understand the relationships between the pH (concentration of H+), H2 concentration, and the ORP measurement. Please note that, in order to simplify this explanation, the presence of other oxidizing redox couples, which also contribute to the final ORP reading, have not been considered.

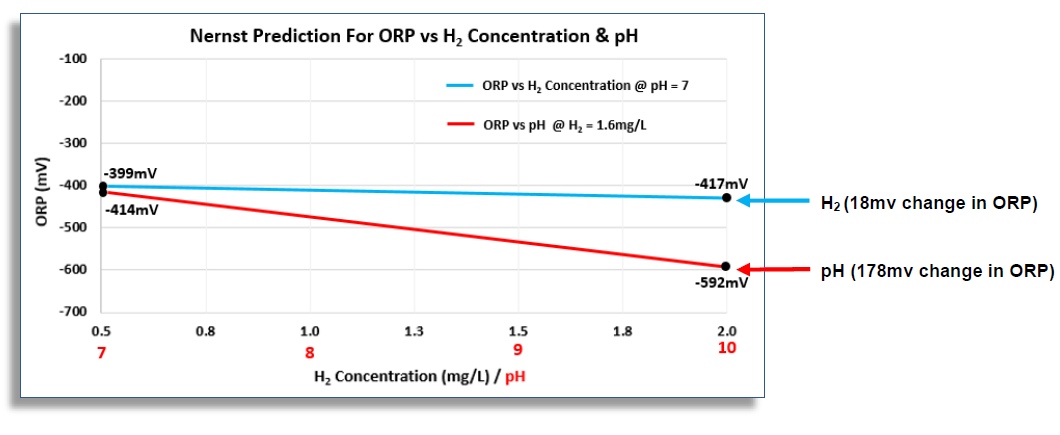
The graph above of ORP (vertical axis) vs both “pH” and “H2 concentration” (horizontal axis) shows that, while the ORP reading is extremely sensitive to changes in pH (red line), it is relatively “insensitive” to changes in dissolved H2 (blue line). Because of this, even small variations in the water’s pH can result in large changes in the ORP reading, masking the relatively small contribution made to the ORP by the dissolved H2. The graphic below views the relative contributions to the ORP value made by pH and H2 in terms of their percentages in another way:
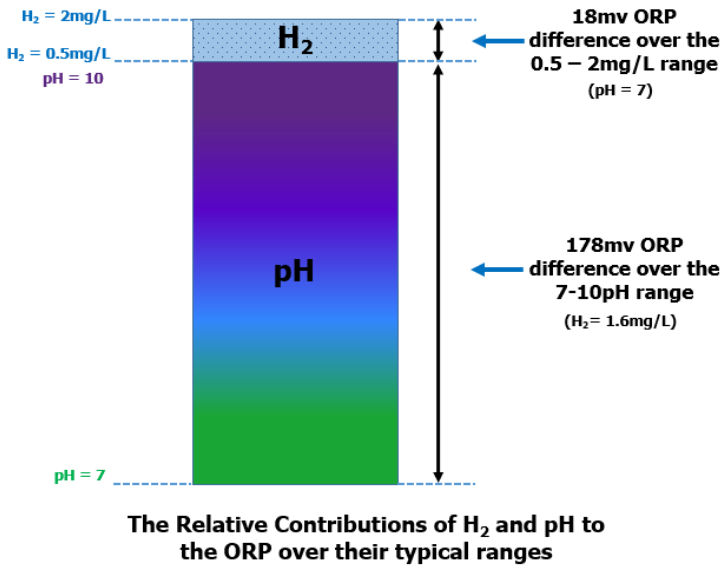
Viewing the relative contributions in this way, it can be seen that, over a typical range in H2 concentration, 0.5 to 2 mg/L, the ORP will only change by a total of 18 mV. Conversely, over a range of 3 pH units, the ORP will change 178 mV. Therefore, over their respective ranges, the pH contributes approximately 90% to the ORP reading, while H2 contributes only about 10%.
Does a “more negative” ORP mean more dissolved H2?
As discussed earlier, the ORP measurement is very sensitive to changes in the water’s pH (concentration of H+) but insensitive to changes in dissolved hydrogen gas. Because ORP is the result of the relative concentrations of two species of hydrogen in the water (H+/H2), a change in the concentration of either species will change the ORP measurement. Therefore, hydrogen water from a neutral-pH hydrogen water device with its much lower average pH will measure a lower-magnitude negative ORP reading than alkaline water, even if it contains twice as much dissolved H2!

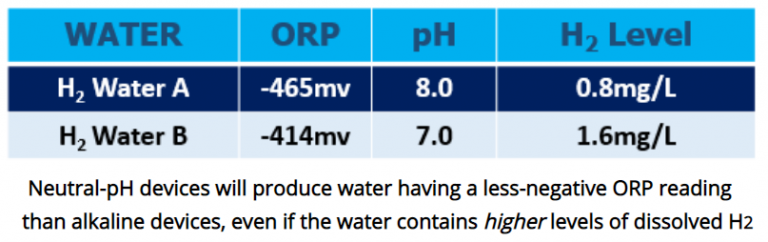
While a negative ORP value may indicate the presence of dissolved hydrogen gas, the ORP measurement cannot be used to measure the level of dissolved hydrogen gas. Therefore, methods that depend on the measurement of the oxidation-reduction potential (ORP) to estimate hydrogen in water, which incorporate electrodes which are not specific to hydrogen, are discouraged. Although a negative ORP is one of the characteristics of hydrogen water, ORP itself does not show the hydrogen concentration. Thus, technologies which rely upon measurement of ORP should not be used as a method to measure the hydrogen concentration.


